Personalized medicine
Personalized (PM), or also referred to as “precision” therapy, represents the highest possible individual approach in the diagnosis, treatment and management of a patient with an oncological disease. It makes it possible to transform healthcare for the need of individual patient diagnosis and treatment (1).
The main goal of PM is to offer the patient a much more precise and targeted diagnosis of his disease and setting the patient up for individual targeted treatment with the highest possible effect and the lowest rate of side effects of the treatment (2).

Individual setting of oncological treatment
For our clients and patients, we bring new possibilities of personalized treatment (individually set for the patient, the so-called «tailored treatment»), which is the result of the latest diagnostic tests and analyzes of the patient’s state of health and his illness.
Personalized therapy represents the highest possible individual approach in the treatment and management of a patient with an oncological disease.
Its biggest advantage is that it eliminates the unwanted effects and lack of effectiveness of conventional chemotherapy or radiotherapy based on general treatment protocols intended for a selected group of patients with a similar disease. However, such treatment does not fully take into account the individuality of the patient, his immune system, metabolic state and the profile of the biological properties of the disease, which are not always analyzed in sufficient detail by common tests used in clinical practice.
By using personalized therapy, we offer our clients the opportunity to receive the best available treatment for their oncological disease. We reduce the risk of undesired effects of non-specific chemotherapy and bring the opportunity to undergo individual, personalized treatment for patients with oncological disease.
The traditional way of treating an oncological patient is the administration of chemotherapy in the case of an indication. These are medicinal substances, the so-called cytostatics, which usually kill all rapidly dividing cells in the patient’s body by disrupting cell division or the process of cell death. Unfortunately, such a method of treatment, based on a combination of one or more cystostatics according to standard protocols (i.e. a combination of drugs and their administration regimens), often leads to many side effects and does not take into account the individual characteristics of the patient, which is reflected in the recurrence of the disease. On the other hand, targeted cancer treatment represents the use of drugs that are precisely indicated for a specific patient with a certain type, characteristics of the disease, based on which these substances are intended for targeted intervention in specific molecules or molecular pathways, cellular processes that stop tumor growth and progression.

The primary goal of targeted, individually set personalized therapy is to effectively act only against cancer cells and to affect non-cancerous cells as little as possible, i.e. not to cause side effects with the administered treatment.
The importance of performing tests for the need for personalized treatment is also evidenced by the fact that already in 2017, up to 76% of oncologists in the USA used NGS (Next Generation Sequencing) analyzes of the disease for the correct individual setting of the patient’s treatment (3), while 60.1% of them were very satisfied with the possibility of using such testing in personalized treatment (4).
It has been proven that patients who undergo the genomic analysis necessary for personalized treatment have a much longer period without symptoms of the disease (without recurrence) as well as overall survival than patients in whom such tests were not performed (5).
This finding also caused a change in the behavior of oncologists in practice, where it is common for up to 65-70% of oncologists to postpone a decision on cancer treatment until the time of receiving the results of molecular tests, while 35-40% of patients will receive treatment other than the one initially planned by the oncologist (6,7). At the same time, these tests can determine for whom (neo)adjuvant chemotherapy is suitable and for whom it is not. That is, information on when chemotherapy will not bring any benefit from the point of view of disease recurrence and survival, it can even be harmful. In practice, the following example is often used: After surgery, oncological treatment is given to a patient who shows a high risk of recurrence on the basis of commonly assessed clinical-pathological parameters, but is ineffective, or it can harm the patient because the patient’s tumor shows genetic characteristics indicating low aggressiveness and risk of recurrence, and thus there is no need to undergo treatment. In medical terminology, such cases are referred to as patients with so-called “high clinical risk and low genomic risk for recurrence” (8,9). Therefore, oncologists consider it important to implement such testing in the decision-making process about the treatment setting for the patient (10,11).

Clinical studies have shown that in up to 18-22% of patients with breast cancer, their clinical-histopathological subtyping was reclassified to a different molecular subtype of the disease, which gave the patients the possibility of specific personalized treatment that was not considered at all before, because their type of malignant disease was considered based on commonly examined parameters for a different type of cancer (12,13).
This molecular classification subsequently enables a better, more effective type of treatment to be selected for the patient and ensures a significantly higher degree of disease response to treatment (14,15). At the same time, it will make it possible to determine which patients are more susceptible to disease recurrence (16).
In some countries, therefore, the use of a multidisciplinary professional team of specialists in the field of personalized therapy is already a matter of course when setting up a patient for his individual treatment (17). They are significantly helped in the decision-making process by the dynamically developing technology of artificial intelligence (Artificial intelligence), which makes it possible to use and evaluate the results of thousands of studies and a meta-analytical approach in the systematic evaluation of treatment options for an oncology patient (18,19).
Websites:
https://www.oncotypeiq.com/en the 21‐gene Recurrence Score (RS; Oncotype DX; Genomic Health, Inc., Redwood City, CA)
https://agendia.com/mammaprint/ the 70‐gene signatures (MammaPrint and BluePrint, respectively; Agendia, Irvine, CA)
https://agendia.com/blueprint/ the 70‐gene and 80‐gene signatures (MammaPrint and BluePrint, respectively; Agendia, Irvine, CA)
https://www.prosigna.com/en/ the 50‐gene Prediction Analysis of Microarrays (PAM50) and PAM50 Risk of Recurrence (PAM50‐ROR; Prosigna; NanoString Technologies, Seattle, WA)
https://myriad-oncology.com/endopredict/ EndoPredict (Myriad Genetics, Inc., Salt Lake City, UT)
https://www.breastcancerindex.com/ Breast Cancer Index (BCI; Biotheranostics, Inc., San Diego, CA)
Watson for Oncology: https://www.youtube.com/watch?v=338CIHlVi7A
Determining the risk of developing the disease
Personalized medicine can also be used in the area of client services, which are aimed at finding patients with a high risk of developing the disease, even before carcinogenesis starts in the patient’s body. Whether it is a patient with a risk of hereditary familial occurrence of a malignant disease in the family, or sporadic types of malignant diseases. In practice, it is primarily genetic testing that helps estimate your chance of developing cancer during your lifetime. The analysis is done by looking for specific changes in your genes, chromosomes or proteins. These changes are called mutations.
Currently available genetic tests are intended for the following types of cancer:
- Breast cancer
- Ovarian cancer
- Colon cancer
- Thyroid cancer
- Prostate cancer
- Pancreatic cancer
- Melanoma
- Sarcoma
- Kidney cancer
- Stomach cancer
Genetic tests can help predict the risk of developing a particular disease (lifelong or short-term, e.g. 5 years). It is only up to each of you, whether you want to find out if you have genes that can cause you to develop cancer and pass such an increased risk of cancer on to your children. In this way, personalized medicine provides you with information that will help you with your health care (20,21).

From a technical point of view, this analysis is primarily about revealing the individual genetic predisposition of an individual to the development of selected cancers based on specific molecular and genetic tests connected with the analysis of possible biological, somatic, familial and environmental risks in the monitored subject. The process of analyzing the risk of developing a malignant disease is supplemented in selected cases with information from previous examinations, e.g. imaging (ultrasound, mammography, CT/MR), histological (reports from a pathologist) and blood tests for the so-called tumor markers.
https://bcrisktool.cancer.gov/
https://cancerscreen.geneplanet.com/en
Diagnosis of the disease at the earliest possible stage (liquid biopsy)
In the field of personalized medicine, we also offer our clients the latest, revolutionary technology (so-called Liquid biopsy), intended for very early diagnosis of the presence of a malignant disease in the patient’s body, long before the disease manifests itself clinically, or is detected on any of the diagnostic examinations. This technology is based on the collection of a minimal amount of biological material from the patient (eg blood, eye fluid, saliva, urine, breast secretion). The obtained sample is sent to selected highly specialized laboratories focused on the molecular diagnosis of cancer diseases.
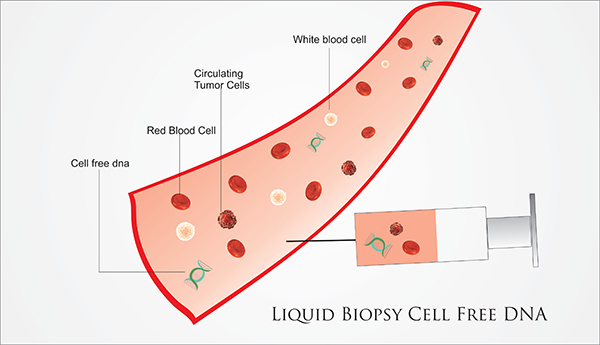
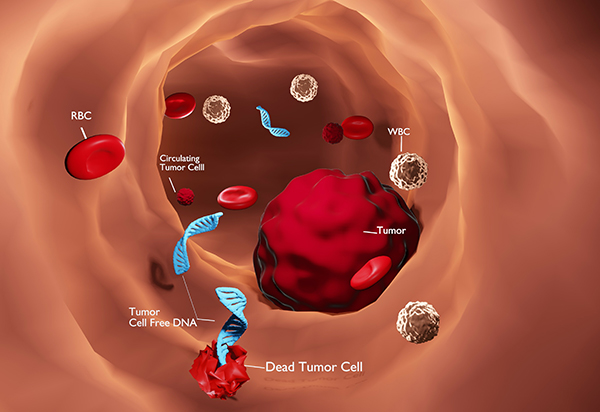
Based on specific diagnostic tests in the field of metabolomics, genomics and proteomics, hundreds to thousands of genes, molecules and their interactions are examined connection From these results, we can determine the presence of the disease in the patient’s body even before the disease is diagnosed by today’s commonly available examinations, such as e.g. USG, X-ray, CT, MMG, MR, blood markers (which is unfortunately often late).
At the same time, the patient has the option in some cases not to undergo painful tissue sampling from the tumor with a biopsy needle, since we can obtain information about the disease in a much more gentle and safe way, precisely with the help of liquid biopsy examination technology. The use of liquid biopsy in the diagnostic process is already part of patient care in many developed countries with a high level of health care (22).
Monitoring the course of the disease
The latest trend and future perspective is the use of liquid biopsy methods in the field of monitoring patients after oncological surgery, i.e. during the administration of oncological treatment and after its completion in the period of long-term multi-year follow-up. In the vast majority, the patient is monitored by his doctor in the form of a clinical examination, blood sampling for low-specific tumor markers, or tests evaluating the side effects of treatment, supplemented by an imaging examination (ultrasound, CT, PET-CT, MR). Unfortunately, such procedures are not always sufficiently sensitive or able to detect early changes in the course of the disease. Here, however, the results of specific genetic, proteomic and metabolomic tests can inform the attending physician much better about the effect / ineffectiveness of the administered chemotherapy / immunotherapy / hormonal treatment compared to a regular clinical examination, (supplemented by blood sampling for tumor markers and/or an imaging examination looking for reduction tumor, possibly the return of the disease in the form of metastases).
Monitoring the behavior of the disease with the help of liquid biopsy method tests makes it possible to detect much earlier that something is “happening” and intervene in the treatment process (change an ineffective, insufficiently effective treatment to another), or predict the return of the disease many months before metastases appear on the control imaging examinations, or become clinically manifest. Such findings give the patient a much greater chance of earlier intervention by the attending physician and thus significantly improve his overall survival.
Course of advanced ovarian cancer with characteristic multiple relapses and therapeutic interventions. Evaluated based on monitoring the levels of the tumor marker Ca-125 (23).
How diagnosis and setting up personalized treatment works
1. Obtaining a sample
Tumor tissue is collected in a special collection set during surgery, or a small part of the tumor is taken from a paraffin block stored in the pathology department after surgery. (We will agree the material collection process with you and your workplace). However, in some cases, only a small amount of your blood sample is needed to extract the DNA, mRNA needed to perform the tests. Samples of biological material will be sent to specialized laboratories in Slovakia, the EU, etc. USA.
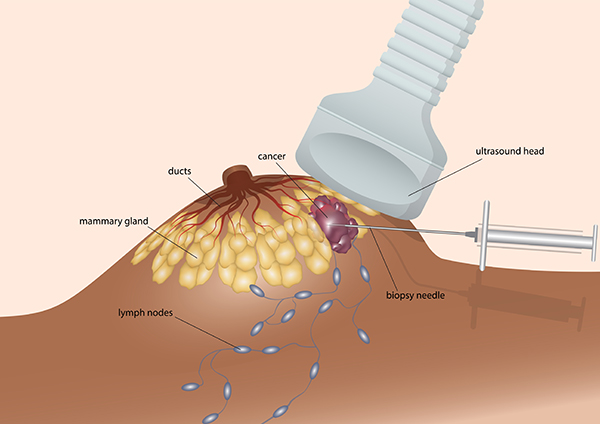
2. Sample analysis
Tissue samples ev. blood is analyzed using the most modern diagnostic technology and highly sophisticated tests aimed at the molecular diagnosis of your disease.


3. Obtaining analysis results and recommendations for targeted treatment
On the basis of diagnostic tests, which are focused on tumor processes of origin, progression, spread of the disease and the response of tumor tissue to treatment, you will receive a professional report on the findings and suggestions for targeted individual treatment, tailored directly to you.
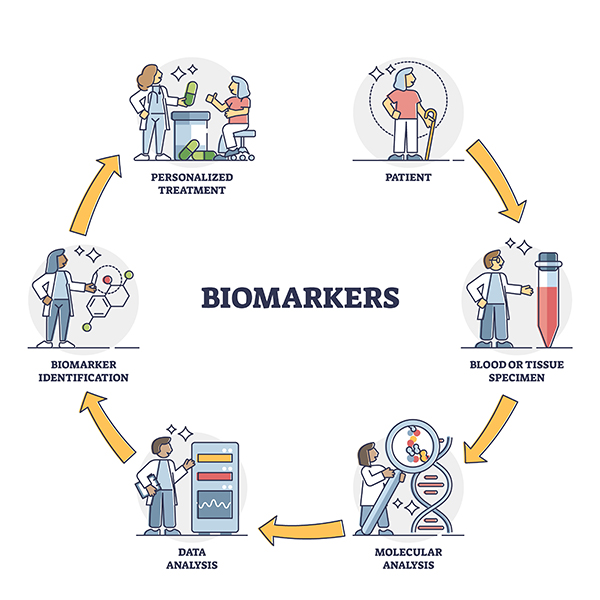
4. Consultation on test results
Our expert in the field of oncology treatment will explain in detail the significance of the results of the personalized medicine diagnostic tests and discuss with you the proposed options for targeted treatment, which we will also send to your treating oncologist if necessary.

Literature:
- Mathur S, Sutton J. Personalized medicine could transform healthcare. Biomed Rep. 2017 Jul;7(1):35.doi:10.3892/br.2017.922.;https://www.ncbi.nlm.nih.gov/pmc/articles/PMC54927/.
- AmericanCancerSociety.Precisionorpersonalizedmedicine. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/precision-medicine.html; Personalizovana liecba zachranuje zivoty pacientov (Aspinall MG, Hamermesh RG. Realizing the Promise of Personalized Medicine. Harvard Bussines Review. October 2007; https://hbr.org/2007/10/realizing-the-promise-of-personalized-medicine.
- Freedman AN, Klabunde CN, Wiant K, Enewold L, Gray SW, Filipski KK, Keating NL, Leonard DGB, Lively T, McNeel TS, Minasian L, Potosky AL, Rivera DR, Schilsky RL, Schrag D, Simonds NI, Sineshaw HM, Struewing JP, Willis G, de Moor JS. Use of Next-Generation Sequencing Tests to Guide Cancer Treatment: Results From a Nationally Representative Survey of Oncologists in the United States. JCO Precis Oncol. 2018 Nov;2:1-13.doi: 10.1200/PO.18.00169. https://pubmed.ncbi.nlm.nih.gov/35135159/.
- de Moor JS, Gray SW, Mitchell SA, Klabunde CN, Freedman AN. Oncologist Confidence in Genomic Testing and Implications for Using Multimarker Tumor Panel Tests in Practice. JCOPrecisOncol.2020Jun11;4:PO.19.00338.doi:10.1200/PO.19.00338. https://pubmed.ncbi.nlm.nih.gov/32923869/.
- Choucair K, Mattar BI, Van Truong Q, Koeneke T, Van Truong P, Dakhil C, Cannon MW, Page SJ, Deutsch JM, Carlson E, Moore DF, Nabbout NH, Kallail KJ, Dakhil SR, Reddy PS. Liquid Biopsy-based Precision Therapy in Patients with Advanced Solid Tumors: A Real-world Experience from a Community-based Oncology Practice. Oncologist.2022Mar11;27(3):183-190.doi:10.1093/oncolo/oyac007.https://pubmed.ncbi.nlm.nih.gov/35274713/.
- Zhu X, Dent S, Paquet L, Zhang T, Tesolin D, Graham N, Aseyev O, Song X. How Canadian Oncologists Use Oncotype DX for Treatment of Breast Cancer Patients. Curr Oncol. 2021 Feb 4;28(1):800-812.doi:10.3390/curroncol28010077. https://pubmed.ncbi.nlm.nih.gov/33557029/;
- Assi HI, Alameh IA, Khoury J, Abdul Halim N, El Karak F, Farhat F, Berro J, Sbaity E, Charafeddine M, Tfayli A, Salem Z, El Saghir N. Impact of Commercialized Genomic Tests on Adjuvant Treatment Decisions in Early Stage Breast Cancer Patients. J Oncol. 2020 Nov 28;2020:9238084. doi: 10.1155/2020/9238084. https://pubmed.ncbi.nlm.nih.gov/33312202/.
- Green N, Al-Allak A, Fowler C. Benefits of introduction of Oncotype DX® testing. Ann R CollSurgEngl.2019Jan;101(1):55-59.doi:10.1308/rcsann.2018.0173. https://pubmed.ncbi.nlm.nih.gov/30322288/;
- Piccart M, van ‘t Veer LJ, Poncet C, Lopes Cardozo JMN, Delaloge S, Pierga JY, Vuylsteke P, Brain E, Vrijaldenhoven S, Neijenhuis PA, Causeret S, Smilde TJ, Viale G, Glas AM, Delorenzi M, Sotiriou C, Rubio IT, Kümmel S, Zoppoli G, Thompson AM, Matos E, Zaman K, Hilbers F, Fumagalli D, Ravdin P, Knox S, Tryfonidis K, Peric A, Meulemans B, Bogaerts J, Cardoso F, Rutgers EJT. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021 Apr;22(4):476-488.doi:10.1016/S1470-2045(21)00007-3. https://pubmed.ncbi.nlm.nih.gov/33721561/.
- Domchek SM, Mardis E, Carlisle JW, Owonikoko TK. Integrating Genetic and Genomic Testing Into Oncology Practice. Am Soc Clin Oncol Educ Book. 2020 May;40:e259-e263. doi: 10.1200/EDBK_280607. https://pubmed.ncbi.nlm.nih.gov/32453613/;
- Thavaneswaran S, Ballinger M, Butow P, Meiser B, Goldstein D, Lin F, Napier C, Thomas D, Best M. The experiences and needs of Australian medical oncologists in integrating comprehensive genomic profiling into clinical care: a nation-wide survey. Oncotarget. 2021 Oct12;12(21):2169-2176.doi:10.18632/oncotarget.28076. https://pubmed.ncbi.nlm.nih.gov/34676049/.
- Whitworth P, Beitsch P, Mislowsky A, Pellicane JV, Nash C, Murray M, Lee LA, Dul CL, Rotkis M, Baron P, Stork-Sloots L, de Snoo FA, Beatty J. Chemosensitivity and Endocrine Sensitivity in Clinical Luminal Breast Cancer Patients in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST) Predicted by Molecular Subtyping. Ann Surg Oncol. 2017 Mar;24(3):669-675.doi:10.1245/s10434-016-5600-x. https://pubmed.ncbi.nlm.nih.gov/27770345/.
- Whitworth P, Stork-Sloots L, de Snoo FA, Richards P, Rotkis M, Beatty J, Mislowsky A, Pellicane JV, Nguyen B, Lee L, Nash C, Gittleman M, Akbari S, Beitsch PD. Chemosensitivity predicted by BluePrint 80-gene functional subtype and MammaPrint in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST). Ann Surg Oncol. 2014 Oct;21(10):3261-7.doi:10.1245/s10434-014-3908-y. https://pubmed.ncbi.nlm.nih.gov/25099655/.
- Beitsch P, Whitworth P, Baron P, Rotkis MC, Mislowsky AM, Richards PD, Murray MK, Pellicane JV, Dul CL, Nash CH, Stork-Sloots L, de Snoo F, Untch S, Lee LA. Pertuzumab/Trastuzumab/CT Versus Trastuzumab/CT Therapy for HER2+ Breast Cancer: Results from the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST). Ann SurgOncol.2017Sep;24(9):2539-2546.doi:10.1245/s10434-017-5863-x.https://pubmed.ncbi.nlm.nih.gov/28447218/.
- Pease AM, Riba LA, Gruner RA, Tung NM, James TA. Oncotype DX® Recurrence Score as a Predictor of Response to Neoadjuvant Chemotherapy. Ann Surg Oncol. 2019 Feb;26(2):366-371.doi:10.1245/s10434-018-071078. https://pubmed.ncbi.nlm.nih.gov/30542840/.
- Sestak I, Buus R, Cuzick J, Dubsky P, Kronenwett R, Denkert C, Ferree S, Sgroi D, Schnabel C, Baehner FL, Mallon E, Dowsett M. Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018 Apr 1;4(4):545-553. doi: 10.1001/jamaoncol.2017.5524. https://pubmed.ncbi.nlm.nih.gov/29450494/.
- Hoefflin R, Geißler AL, Fritsch R, Claus R, Wehrle J, Metzger P, Reiser M, Mehmed L, Fauth L, Heiland DH, Erbes T, Stock F, Csanadi A, Miething C, Weddeling B, Meiss F, von Bubnoff D, Dierks C, Ge I, Brass V, Heeg S, Schäfer H, Boeker M, Rawluk J, Botzenhart EM, Kayser G, Hettmer S, Busch H, Peters C, Werner M, Duyster J, Brummer T, Boerries M, Lassmann S, von Bubnoff N. Personalized Clinical Decision Making Through Implementation of a Molecular Tumor Board: A German Single-Center Experience. JCO Precis Oncol. 2018 Aug16;2:PO.18.00105.doi:10.1200/PO.18.00105. https://pubmed.ncbi.nlm.nih.gov/32913998/.
- Jie Z, Zhiying Z, Li L. A meta-analysis of Watson for Oncology in clinical application. Sci Rep.2021Mar11;11(1):5792.doi:10.1038/s41598-021-84973-5. https://pubmed.ncbi.nlm.nih.gov/33707577/.
- Kim MS, Park HY, Kho BG, Park CK, Oh IJ, Kim YC, Kim S, Yun JS, Song SY, Na KJ, Jeong JU, Yoon MS, Ahn SJ, Yoo SW, Kang SR, Kwon SY, Bom HS, Jang WY, Kim IY, Lee JE, Jeong WG, Kim YH, Lee T, Choi YD. Artificial intelligence and lung cancer treatment decision: agreement with recommendation of multidisciplinary tumor board. Transl Lung CancerRes.2020Jun;9(3):507-514.doi:10.21037/tlcr.2020.04.11. https://pubmed.ncbi.nlm.nih.gov/32676314/.
- Pashayan N, Antoniou AC, Ivanus U, Esserman LJ, Easton DF, French D, Sroczynski G, Hall P, Cuzick J, Evans DG, Simard J, Garcia-Closas M, Schmutzler R, Wegwarth O, Pharoah P, Moorthie S, De Montgolfier S, Baron C, Herceg Z, Turnbull C, Balleyguier C, Rossi PG, Wesseling J, Ritchie D, Tischkowitz M, Broeders M, Reisel D, Metspalu A, Callender T, de Koning H, Devilee P, Delaloge S, Schmidt MK, Widschwendter M. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol. 2020; 17(11): 687–705. Published online 2020 Jun 18. doi: 10.1038/s41571-020-0388-9.Correctionin: NatRevClinOncol.2020;17(11):716.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7567644/.
- Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, Lipkin SM, Syngal S, Wollins DS, Lindor NM. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol. 2015; 33(31):3660-3667,http://acmgen.org/wp-content/uploads/2017/04/Genetic-and-genomic-testing-for-cancer-susceptibility-ASCO-2015.pdf.
- Kamps R, Brandão RD, Bosch BJ, Paulussen AD, Xanthoulea S, Blok MJ, Romano A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int J Mol Sci. 2017 Jan 31;18(2):308. doi: 10.3390/ijms18020308. https://pubmed.ncbi.nlm.nih.gov/28146134/.
- du Bois A, Pfisterer J. Primärtherapie des Ovarialkarzinoms [First-line therapy in ovarian cancer]. Zentralbl Gynakol. 2004 Oct;126(5):312-4. German. doi: 10.1055/s-2004-820390.

